UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM
Current Report Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event
Reported):
(Exact Name of Registrant as Specified in its Charter)
| (State or Other Jurisdiction of | (Commission File Number) | (I.R.S. Employer Identification | ||
| Incorporation) | Number) |
(
(Addresses, including zip code, and telephone numbers, including area code, of principal executive offices)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Trading Symbol(s) | Name of each exchange
on which registered: | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01. | Regulation FD Disclosure. |
The information in this Current Report (including Exhibit 99.1) is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section. The information in this Current Report (including Exhibit 99.1) shall not be incorporated by reference into any registration statement or other document pursuant to the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
Members of the Theravance Biopharma, Inc. management team will be participating in presentations at (i) the Morgan Stanley 18th Annual Global Healthcare Conference 2020 on September 14, 2020 and (ii) the H.C. Wainwright 22nd Annual Global Investment Conference on September 15, 2020. A copy of the Company’s corporate overview is being furnished pursuant to Regulation FD as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits.
| 99.1 | Slide deck entitled Corporate Overview - dated September 2020 | |
| 104 | Cover Page Interactive Data File (cover page XBRL tags embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| THERAVANCE BIOPHARMA, INC. | ||
| Date: September 14, 2020 | By: | /s/ Andrew Hindman |
| Andrew Hindman | ||
| Senior Vice President and Chief Financial Officer | ||
Exhibit 99.1

Corporate Overview September 2020 THERAVANCE BIOPHARMA ® , THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries). All third party trademarks used herein are the property of their respective owners. © 2020 Theravance Biopharma. All rights reserved.

Forward - looking statements 2 Under the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , the company cautions investors that any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward - looking statements or projections . Examples of forward - looking statements in this presentation may include the Company’s strategies, plans and objectives, the Company’s regulatory strategies and timing of clinical studies (including the data therefrom), the potential characteristics, benefits and mechanisms of action of the Company’s product and product candidates, the potential that the Company’s research programs will progress product candidates into the clinic, the Company’s expectations for product candidates through development, the Company's expectations regarding its allocation of resources, potential regulatory approval and commercialization (including their differentiation from other products or potential products), product sales or profit share revenue and the Company’s expectations for its 2020 operating loss, excluding share - based compensation and other financial results . The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to the impacts on the COVID - 19 global pandemic on our business, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s compounds or product candidates are unsafe or ineffective, risks that product candidates do not obtain approval from regulatory authorities, the feasibility of undertaking future clinical trials for our product candidates based on policies and feedback from regulatory authorities, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, current and potential future disagreements with Innoviva, Inc . and TRC LLC, the uncertainty of arbitration and litigation and the possibility that an arbitration award or litigation result could be adverse to the Company . Other risks affecting Theravance Biopharma are in the company's Form 10 - Q filed with the SEC on August 10 , 2020 , and other periodic reports filed with the SEC .

Creating transformational value for stakeholders 1. Cash, cash equivalents and investments as of 6/30/2020. 2. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by Glaxo Group Limited ("GSK") (net of Theravance Respiratory Company (“TRC”) expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agre eme nt over the next four fiscal quarters). 75% of royalties received pledged to service PhaRMA SM notes, 25% of royalties received retained by TBPH. All statements concerning TRELGY ELLIPTA based on publicly available infor mat ion. 3 Innovative and productive research engine feeding pipeline of organ - selective molecules designed to optimize therapeutic index Proven development expertise and established commercial infrastructure with strong history in respiratory Strategic partnerships complement internal capabilities and balance technical, execution and financial risks Strong capital position of $438.3m in cash 1 augmented by TRELEGY ELLIPTA 2 royalties and YUPELRI ® launch Multiple milestones and value driving catalysts in 2020, 2021 and beyond

Our science Organ - selective molecules designed to optimize therapeutic index
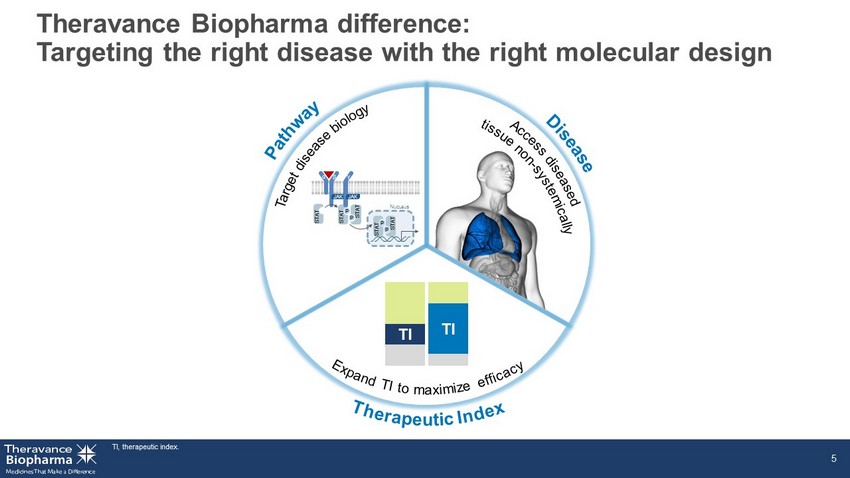
Theravance Biopharma difference: Targeting the right disease with the right molecular design TI, therapeutic index. 5 TI TI α STAT P STAT P STAT JAK β γ JAK N UCLEUS P STAT P STAT

Research and development portfolio of designed molecules: brain, lung, GI and eye 6 Molecular Design Biology Medicinal Chemistry DMPK Development and Commercial Symptomatic nOH (Neurogenic orthostatic hypotension) Asthma Lung Transplant Rejection IPF (idiopathic pulmonary fibrosis) COPD (chronic obstructive pulmonary disorder) — APPROVED COVID - 19 CD (Crohn’s disease) UC (ulcerative colitis) Celiac Disease DME (diabetic macular edema) DMPK, drug metabolism and pharmacokinetics; GI, gastrointestinal.

Program Indication Research Phase 1 Phase 2 Phase 3 Filed Marketed Collaborator Ampreloxetine (TD - 9855) NRI S ymptomatic nOH Wholly - owned Organ - Selective TD - 1473 GI JAKi UC CD TD - 5202 Irreversible JAK3i Inflammatory intestinal diseases YUPELRI ® (revefenacin) LAMA COPD TD - 8236 Inhaled JAKi Asthma Wholly - owned TD - 0903 Inhaled JAKi COVID - 19 Program Indication Research Phase 1 Phase 2 Phase 3 Filed Marketed Rights Economic Interests TRELEGY ELLIPTA 1 FF/UMEC/VI COPD GSK & Innoviva, Inc. Asthma Skin - selective JAKi Dermatological diseases Key programs supported by proven development and commercial expertise 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties received pledged to service outstanding notes, 25% of royalti es received retained by TBPH. All statements concerning TRELEGY ELLIPTA based on publicly available information. FF/UMEC/VI, fluticasone/umeclidinium/vilanterol; JAKi, Janus kinase inhibitor; LAMA, long act ing muscarinic receptor antagonist; NRI, norepinephrine reuptake inhibitor. 7 Phase 2 Phase 2 Phase 2b/3 Phase 1 Phase 3 Marketed Marketed Research Phase 2 Marketed

YUPELRI ® (revefenacin) inhalation solution First and only once - daily, nebulized maintenance medicine for COPD

YUPELRI ® (revefenacin) inhalation solution FDA - approved for the maintenance treatment of COPD 1. Global Strategy for Diagnosis, Management, and Prevention of COPD, 2018; 2. TBPH market research (N = 160 physicians); ref ers to US COPD patients 3. Loh CH, et al. Ann Am Thorac Soc. 2017 Aug;14:1305 - 11. LAMA, long acting muscarinic receptor antagonist; PIFR, peak inspiratory flow rate. 9 9 Once - daily LAMAs are first - line therapy for moderate - to - severe COPD 1 9% of COPD patients (~800,000) use nebulizers for ongoing maintenance therapy; 41% use nebulizers at least occasionally for bronchodilator therapy 2 Nebulized therapy associated with reduced hospital readmissions in low PIFR patients 3 Companies copromote under US profit/loss share TBPH and MYL worldwide strategic collaboration to develop and commercialize nebulized YUPELRI ® ( revefenacin ) 65% 35% TBPH MYL

Majority of YUPELRI ® volume flows through durable medical equipment channel (approximately 3 - month lag in data capture); remaining volume flows thro ugh hospitals, retail and long - term care pharmacies. Wholesale acquisition cost (WAC): $1,066 per month (or ~$35 per day). 1. As of June 30, 2020. 2. TBPH estimate de riv ed from integrating multiple data sources. 3. For patients with supplemental insurance; approximately 20% of patients may be responsible for co - pay and/or supplemental insurance. Source: www. CMS.gov. 10 181 wins (equates to 329 accounts) ~86 reviews scheduled (>456 potential accounts) 100% medical support requests fulfilled <30 days FORMULARY 1 Field force productivity goals exceeded ~44,000 patients 2 prescribed (through Q2 2020) PATIENT 100% Medicare Part B 3 72% of commercial payer lives covered (comprises ~8% of the YUPELRI ® business) ACCESS YUPELRI ® launch metrics Strong customer acceptance and market uptake

Inhaled JAK inhibitor Portfolio Potential to transform treatment of respiratory inflammation
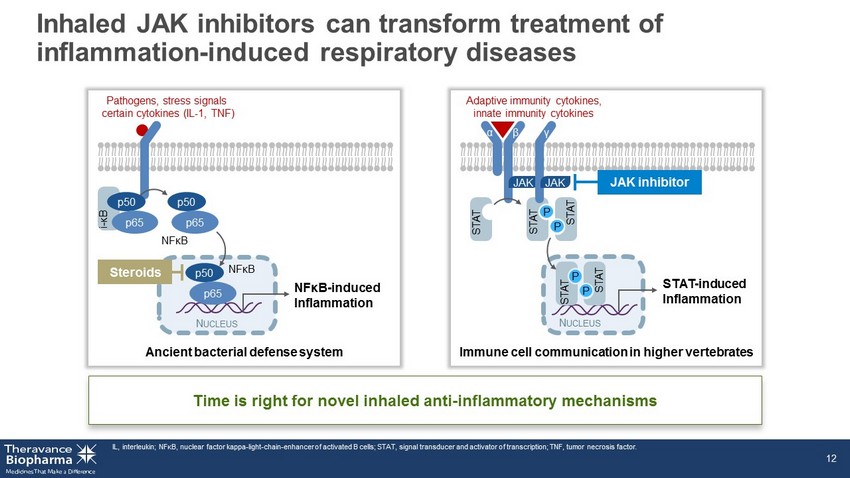
Inhaled JAK inhibitors can transform treatment of inflammation - induced respiratory diseases IL, interleukin; NF κ B, nuclear factor kappa - light - chain - enhancer of activated B cells; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor. 12 Time is right for novel inhaled anti - inflammatory mechanisms Ancient bacterial defense system Pathogens, stress signals certain cytokines (IL - 1, TNF) N UCLEUS NFκB - induced Inflammation p50 p65 p50 p65 i - κB NFκB p50 p65 NFκB Steroids Adaptive immunity cytokines, innate immunity cytokines Immune cell communication in higher vertebrates STAT - induced Inflammation α N UCLEUS STAT P STAT P STAT P STAT P STAT JAK β γ JAK JAK inhibitor

TD - 8236 Potential first inhaled JAKi for asthma

High medical and economic burden in uncontrolled asthma *Asthma that requires high - dosage ICS + LABAs to prevent the disease from being uncontrolled) or asthma that remains uncontrolle d despite treatment. 1. World Health Organization; 2. https://www.aafa.org/asthma - facts/; 3. Sadatsafavi, M., et al. Can Respir J 2010;17:74 - 80. ICS, inhaled corticosteroids; IFN, interferon; LABA, long - acting β2 agonists; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; T2, type 2; TSLP, thymic stromal lymphopoietin. 14 16 14 Severe* Moderate US asthma market (May 2020) 61 25 US cases 8% of adults 8% of children 2 Healthcare utilization 3 25M ~$15B P ATIENT P OPULATION C URRENT T REATMENT L ANDSCAPE S TRATEGIC O PPORTUNITY TD - 8236 Potential to transform the treatment of respiratory inflammation by treating moderate - to - severe asthma regardless of T2 phenotype, including patients who remain symptomatic despite compliance on high - dose ICS ICS + LABA (often fail to control disease) Approved biologics (affect subsets of patients) US medical costs ~$58B T2 - high T2 - low IL - 4 IL - 23 /IL - 12 IL - 13 IL - 6 IL - 5 IL - 27 TSLP IFN - γ Bold: biologics in development or approved. JAK/STAT cytokines implicated in moderate - to - severe asthma • XOLAIR (omalizumab) • NUCALA (mepolizumab) • CINQAIR (reslizumab) • FASENRA (benralizumab) • DUPIXENT (dupilumab) Step - up for severe asthma: LTRAs, tiotropium, OCS, biologics cases worldwide 1 339M

TD - 8236: Phase 1 clinical trial design Parts A & B completed September 2019; Part C data expected 4Q 2020 PART C MoA Biomarkers (Moderate - to - severe asthmatics) • MoA biomarkers to be assessed in target patient population • Goal: build confidence in compound, MoA and dose in early - development FeNO, fractional exhaled nitrous oxide; MAD, multiple ascending dose; SAD, single ascending dose. 15 4000µg N = 16 active/8 Placebo Study open and enrolling All cohorts: N = 6 active/2 Placebo 4500µg 500µg 1500µg 150µg 50µg 1 2 3 4 5 6 7 D 1500 µg 1 2 3 4 5 6 7 D 150µg 500µg 1500µg 1 2 3 4 5 6 7 D 1 2 3 4 5 6 7 D 1 2 3 4 5 6 7 D PART A PART B SAD ( Healthy volunteers) Safety and PK MAD ( Mild asthmatics) Safety, PK, and PD (FeNO)

TD - 8236: Lung - selective pan - JAK inhibitor Phase 2 allergen challenge study 16 Phase 2 allergen challenge study underway Data expected 4 th Q 2020 TD - 8236 Phase 2 Lung Allergen Challenge 12 weeks (N=21) Dose characterization Randomized, double - blind, placebo - controlled, crossover study

TD - 0903 Program Nebulized lung - selective pan - JAK inhibitor to treat: • Acute hyperinflammation of the lung in COVID - 19 • Chronic inflammation for the prevention of lung transplant rejection

Leveraging respiratory expertise for potential acute treatment in response to a global pandemic 1. https://coronavirus.jhu.edu/map.html , number as of 9/10; 2. IHME. 18 US patients 1 >6M patients become hospitalized 2 ~2.4% No vaccine available Current treatment: Supportive therapy As of July 1, 2020: 439 drugs in 2327 trials worldwide S TRATEGIC O PPORTUNITY TD - 0903 Inhaled lung - specific therapeutic: potential to be used in combination with other treatment modalities (e.g., antivirals) to provide additional therapeutic benefit without risk of systemic immunosuppressive issues that may occur with systemic anti - inflammatories P ATIENT P OPULATION C URRENT T REATMENT L ANDSCAPE patients worldwide 1 >27.8M

Potential for TD - 0903 effect Host inflammatory response to COVID - 19 drives ALI and ARDS Adapted from Siddiqi HK, et al. J Heart Lung Transplant 2020 Mar 20. CRP, C - reactive protein; LDH, lactate dehydrogenase; NT - proBNP, ventricular natriuretic peptide; SIRS, severe inflammatory respo nse syndrome. 19 19 Clinical symptoms Clinical signs Potential therapies Mild constitutional symptoms Fever >99.6 ° F Dry cough, diarrhea, headache Shortness of breath Hypoxia (PaO 2 /FiO 2 ≤300 mmHg) Lymphopenia, increased prothrombin time, increased D - Dimer and LDH (mild) Abnormal chest imaging Transaminitis Low normal procalcitonin ARDS SIRS/shock Cardiac failure Elevated inflammatory markers (CRP, LDH, IL - 6, D - dimer, ferritin) Troponin, NT - proBNP elevation Time Course IIA IIB Corticosteroids, human immunoglobulin, Anti - IL - 6; other single pathway inhibitors, JAK inhibitors Remdesivir, chloroquine, hydroxychloroquine, convalescent plasma transfusions Reduce immunosuppression Severity of Illness Stage I (Early Infection) Stage II (Pulmonary Phase) Stage III (Hyperinflammation Phase) Viral response phase Host inflammatory response phase

TD - 0903: Development plan designed to progress rapidly SOC, standard of care. 20 Phase 1: Healthy volunteers Dose A Dose B Dose C Phase 2: Hospitalized COVID - 19 patients Dose A Dose B Dose C Single - ascending dose Multiple - ascending dose x 7 days Dose X + SOC Placebo + SOC x x Part 2: Parallel group x 7 days Dose A Dose B Dose C Repeat - dose safety, tolerability & PK Single - dose safety, tolerability & PK Repeat - dose safety, tolerability, PK & early dose - dependent efficacy Proof of concept efficacy and safety Part 1: Multiple - ascending dose x 7 days

S TRATEGIC O PPORTUNITY First - in - disease opportunity for the prevention of lung transplant rejection 21 1. http://www.transplant - observatory.org/data - charts - and - tables/ ; 2. United Network for Organ Sharing (UNOS), https://unos.org/data/transplant - trends; 3. Chambers DC et al, JHLT 2018; 37(10): 1169 - 1183. CAGR, compound annual growth rate; CF, cystic fibrosis; CLAD, chronic lung allograft dysfunction; mAb, monoclonal antibody; M MF, mycophenolate mofetil. lung transplants per year in US 2 2,714 TD - 0903 Potential first approved therapy specifically to prevent acute lung transplant rejection and development of CLAD Use following lung transplantation could improve patient morbidity and mortality risk, and reduce need for re - transplantation Lung transplants have the poorest prognosis of all solid organ transplants COPD, IPF, and CF top 3 diagnoses driving need for lung transplantation CAGR since 1988 15% mortality at 6 years post transplant 3 ~50% • Calcineurin inhibitors (tacrolimus) • Anti - proliferative agents (MMF) No FDA - approved therapies to prevent lung transplant rejection or CLAD Current standard of care: triple immunosuppression therapy medical/productivity costs (2015 – 2025) $3.5B P ATIENT P OPULATION C URRENT T REATMENT L ANDSCAPE • Corticosteroids • IL - 2 mAb induction therapy (basiliximab) lung transplants worldwide, 2019 1 6,240

Month 6 0 5 10 15 20 F i r s t B P A R R a t e ( % ) Pan - JAK inhibitors can prevent transplant rejections Noninferiority trial of tofacitinib vs cyclosporine (CsA) in kidney transplant recipients 1 ‣ JAK inhibition was superior to cyclosporine in prevention of acute and chronic rejections ‣ Serious infections increased with systemic JAK inhibitors including CMV 1. Vincenti F, et al. Am J Transplant 2015;15:1644 - 53. *p<0.001 vs CsA . BPAR, biopsy - proven acute rejection; CMV, cytomegalovirus; SE, standard error; TWC2, time - weighted 2 - h post - dose concentrations . 22 Chronic Rejection Tofacitinib is superior to CsA in efficacy measures Acute Rejection Increased infection risk with tofacitinib over CsA CsA Tofacitinib 50 40 30 20 10 0 Month 12 * IF/TA % 24 5 53 25 0 20 40 60 Tofacitinib CsA CMV disease Serious infection 12 - month Kaplan - Meier estimates, % (SE)

Ampreloxetine (TD - 9855) Once - daily norepinephrine reuptake inhibitor to treat symptomatic neurogenic orthostatic hypotension

Reduced quality of life, significant care - giver burden and limited therapeutic options for symptomatic nOH patients 24 1. Mathias C, et al. J Neurol 1999;246:893 - 8; 2. Ha AD, et al. Parkinsonism Relat Disord 2011;17:625 - 8; 3. Not all patients are treated with prescription medication P ATIENT P OPULATION US patients ~350K Current treatments (midodrine, fludrocortisone, droxidopa) have significant limitations Subset of patients do not respond None demonstrate durable effect Safety profiles that limit use Require multiple daily dosing nOH is a symptom of MSA, PAF and PD 70 – 80% of MSA patients 1 , and 30 – 50% of PD patients 2 have nOH 3 S TRATEGIC O PPORTUNITY Ampreloxetine Designed to reduce symptoms of nOH by prolonging the effect of endogenous norepinephrine with the potential to provide a meaningful and durable symptom improvement to underserved patients C URRENT T REATMENT L ANDSCAPE

Untreated nOH NE Release at Neurovascular Junction Systolic Blood Pressure Designed to reduce symptoms of nOH by prolonging the effect of endogenous norepinephrine 1. Palma JA, Kaufmann H. Mov Disord Clin Pract 2017;4:298 - 308. NE, norepinephrine; NET, norepinephrine transporters. 25 Vasodilation Blood pressure Vasoconstriction Blood pressure Syncope Normal x Increase standing blood pressure x Increased brain profusion x Reduce symptoms of symptomatic nOH 1 NE A XON TERMINAL D ENDRITE + Ampreloxetine Ampreloxetine Reduction in syncope Normal

Ampreloxetine: Potential to provide meaningful and durable symptom improvement to underserved patients Baseline OHSA #1 (Orthostatic Hypotension Symptom Assessment Question 1) >4 points. Negative change indicates improvement in symptoms; improvement of 1 point is defined as the MCID (minimal clinically importan t d ifference). ITT, intention - to - treat; SD, standard deviation. Mean (SD) change from baseline in OHSA #1 score n=17 n=13 n=7 n=6 Week Study 169: 4 weeks (N=188) Randomized, double - blind, placebo - controlled, parallel group Study 170: 22 weeks (N=254) Randomized 6 - week withdrawal phase Phase 3 registrational program ongoing; 4 - week efficacy data expected 2021 26 Phase 3 Registrational Program Ampreloxetine Phase 2 data in nOH; 20 weeks of treatment Extension study: 3 years Completers: Considered clinically meaningful Withdrawal Durability Efficacy -7 -6 -5 -4 -3 -2 -1 0 1 2 3 0 4 8 12 16 20 24

TD - 1473 (JNJ - 8398) Oral gut - selective pan - JAK inhibitor to treat inflammatory bowel diseases

Need for new medicines to treat Inflammatory Bowel Disease 28 HRQoL, health - related quality of life; IBD, inflammatory bowel disease. 1. GBD 2017 Inflammatory Bowel Disease Collaborators. Lancet 2020;5:17 - 30; 2. https://www.crohnscolitisfoundation.org/sites/default/files/2019 - 02/Updated%20IBD%20Factbook.pdf P ATIENT P OPULATION US disease burden 2 $31B Standard of care: Biologics have become the mainstay of treatment in moderate - to - severe patients CAGR 2018 – 2026 ~4.4% global cases, 2017 1 6.8M global IBD treatment market, 2018 $16B Current US patients Steroids, immunosuppressants, and TNF inhibitors associated with side effects that further decrease HRQoL S TRATEGIC O PPORTUNITY TD - 1473 Gut - selective agent: if used earlier in the course of disease, has potential to be a new cost - effective therapy option that reduces associated disease management costs and improves patient HRQoL current US patients 2 1.6M C URRENT T REATMENT L ANDSCAPE 780K CD cases 907K UC cases

STAT - induced Inflammation α N UCLEUS STAT P STAT P STAT P STAT P STAT JAK β γ JAK JAK inhibitor JAK - STAT pathway: orchestrating signaling of multiple pro - inflammatory cytokines Clark JD, et al. J Med Chem 2014; 57:5023 - 5038. EPO, erythropoietin; GM - CSF, granulocyte - macrophage colony - stimulating factor; Tyk, tyrosine kinase. 29 γ c cytokines (IL - 2, IL - 4, IL - 7, IL - 9, IL - 15, IL - 21) Type 1 IFNs, IL - 10 family IL - 6, IL - 11, IL - 13, IL - 27, IL - 31, IL - 35 IFN γ IL - 12, IL - 23 EPO, TPO GM - CSF, IL - 3, IL - 5 α JAK1 β γ JAK3 α JAK1 β γ Tyk2 α JAK1 β γ JAK2 Tyk2 α JAK1 β γ JAK2 α JAK2 β γ Tyk2 α JAK2 β γ JAK2

TD - 1473 is an oral, gut - selective pan - JAK inhibitor Preclinical data package for TD - 1473 represents a potential breakthrough approach to the treatment of IBD Journal of Crohn’s & Colitis April 20, 2020 30 Gut selectivity Potent inhibition of Tyk2 Anti - inflammatory activity in disease model x x Lumen Vehicle treated control TD - 1473 treatment x 7.5 8 8.5 9 9.5 10 Tofacitinib TD-1473 Inhibitory potency (pKi) Tyk2

Systemic exposures low; tissue concentrations at or above JAK inhibition levels *Tofacitinib concentrations extracted from J Pharmacol Exp Ther 348:165 – 173, January 2014. BID, twice daily; IC 50 , concentration to produce 50% maximal inhibition; PK, pharmacokinetics. 31 Tofacitinib 10 mg BID* TD - 1473 20 mg TD - 1473 80 mg TD - 1473 270 mg Colonic Tissue Concentrations of TD - 1473 TD - 1473 Dose 1 10 100 1000 10,000 20 mg 80 mg 270 mg Range of JAK IC 50 values TD - 1473 Total Concentration (nM) Plasma Concentrations in UC Patients Time (h) 100 200 300 0 0 1 2 3 4 Plasma Concentration (nM)

TD - 1473: Gut - selective pan - JAK inhibitor Late - stage studies in Crohn’s disease and ulcerative colitis Phase 2 Crohn’s and Phase 2b/3 ulcerative colitis studies ongoing Phase 2 Crohn’s and Phase 2b ulcerative colitis data expected 2021 Global collaboration with Janssen leverages joint development expertise and provides significant economics to TBPH 2 1. Maintenance phase of the study will have induction responder patients re - randomized to active doses compared to placebo at 4 4 weeks. 2. Deal value up to $1B in payments to TBPH, including $100M upfront; profit - share in US (33% TBPH, 67% Janssen); double - digit r oyalties to TBPH ex - US. Crohn’s disease Ulcerative colitis Phase 2: 12 weeks (N=160) Dose - finding induction Active treatment extension: 48 weeks Phase 2b/3: 8 weeks (N=240) Dose - finding induction Maintenance phase 1 : 44 weeks Phase 3: 8 weeks (N=640) Dose - confirming induction Responders 32

TD - 5202 Organ - gut selective irreversible JAK3 inhibitor to treat inflammatory intestinal diseases

Celiac disease has no current treatments and serious health consequences 34 1. 1% prevalence, BeyondCeliac.org; 2. 2018 US population 327M Census.gov; 3. Theravance Market Research; S TRATEGIC O PPORTUNITY P ATIENT P OPULATION US patients 1,2 3.3M No approved treatment Only available intervention is strict life - long gluten - free diet 30% of diagnosed patients are poorly controlled despite best dietary efforts 3 TD - 5202 Organ - gut selective irreversible JAK3 inhibitor: potential to deliver significant value for both patients and payers increase in US over past 50 y 4 – 4.5x Global prevalence 1% higher healthcare costs than controls >2x C URRENT T REATMENT L ANDSCAPE Celiac Normal

JAK3 - dependent cytokines play central role in pathogenesis of celiac disease ‣ Proof - of - relevance from recent positive Phase 2 data with systemic JAK3 inhibitor in alopecia areata, another T - cell mediated disease ‣ Localized JAK3 inhibition important to avoid systemic immunosuppression (genetic JAK3 deficiency leads to severe immunodeficiency) Figure adapted from Jabri B and Sollid L. J Immunol 2017;198:3005 - 14. IE - CTL, intraepithelial cytotoxic lymphocyte; IEL, intraepithelial lymphocyte. 35 IL - 2, IL - 4, IL - 7, IL - 9, IL - 15, IL - 21 I MMUNE C ELL CD Pathogenesis α N UCLEUS STAT P STAT P STAT P STAT P STAT JAK1 β γ JAK3 P P P Gluten peptides G LUTEN - S PECIFIC T H 1 CELLS I NTESTINAL E PITHELIUM L AMINA P ROPIA I NTESTINAL L UMEN IFNγ IL - 2 IL - 21 IEL IE - CTL IL - 15
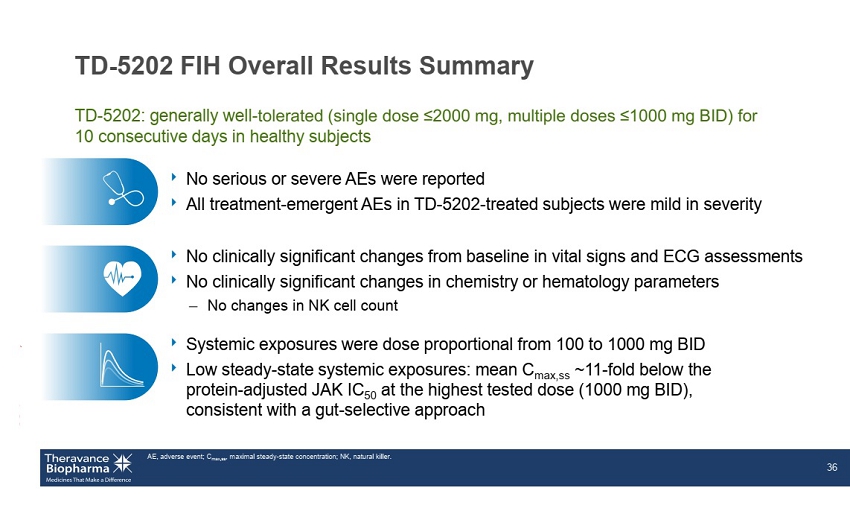
TD - 5202 FIH Overall Results Summary ‣ No serious or severe AEs were reported ‣ All treatment - emergent AEs in TD - 5202 - treated subjects were mild in severity AE, adverse event; C max,ss , maximal steady - state concentration; NK, natural killer. 36 TD - 5202: generally well - tolerated (single dose ≤2000 mg, multiple doses ≤1000 mg BID) for 10 consecutive days in healthy subjects ‣ Systemic exposures were dose proportional from 100 to 1000 mg BID ‣ Low steady - state systemic exposures: mean C max,ss ~11 - fold below the protein - adjusted JAK IC 50 at the highest tested dose (1000 mg BID), consistent with a gut - selective approach ‣ No clinically significant changes from baseline in vital signs and ECG assessments ‣ No clinically significant changes in chemistry or hematology parameters – No changes in NK cell count

Economic interest GSK’s TRELEGY ELLIPTA (FF/UMEC/VI): First and only once - daily single inhaler triple therapy

Economic interest in GSK’s TRELEGY Upward - tiering royalties of ~5.5 – 8.5% of worldwide net sales 1 Q2 net sales of £194m (or $241M) Grew market share with sales up 58% year - over - year Asthma sNDA approved September 9, 2020 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties pledged to service outstanding notes, 25% of royalties retained by TBPH. Our non - recourse Triple II 9.5% Fixed Rate Term Notes due on or before 2035. All statements concerning TRELEGY based on publicly available information. TRELEGY is FF/UMEC/VI or fluticasone furoate/umecl idi nium/vilanterol; comprised of ICS, LAMA, and LABA, active components of Anoro (UMEC/VI). 38 TRELEGY BREO asthma sNDA approved

The Theravance Biopharma Difference

GSK’s TRELEGY ELLIPTA 1 ‣ Asthma sNDA asthma approved September 9, 2020 TD - 8236 ‣ Phase 1 Part C data in severe asthmatics ‣ Phase 2 allergen challenge data Ampreloxetine ‣ Phase 3 4 - week efficacy data TD - 1473 ‣ Phase 2b/3 ulcerative colitis topline data ‣ Phase 2 Crohn’s topline data Multiple potential milestones and value - driving catalysts expected in 2020, 2021 and beyond 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties received pledged to service outstanding notes, 25% of royalti es received retained by TBPH. All statements concerning TRELEGY ELLIPTA based on publicly available information. 40 TD - 0903 ‣ Phase 1 study in healthy volunteers in the UK ‣ Phase 2 study in hospitalized patients with COVID - 19 in the UK – Part 2: multi - center study conducted at hospital - based clinical sites globally, including the U.S. 2020 2021 Commercial progression of YUPELRI ® and GSK’s TRELEGY ELLIPTA TD - 5202 ‣ Phase 1 topline data

Creating transformational value for stakeholders 41 Innovative research yielding organ - selective molecular designed assets Proven development and commercial expertise Strategic partnerships Value driving catalysts Strong capital position

Holding steadfast to our mission 42 …treatments for serious diseases to maximize patient benefit while minimizing patient risk Discovering… Developing… Commercializing…

About YUPELRI ® (revefenacin) inhalation solution YUPELRI ® (revefenacin) inhalation solution is a once - daily nebulized LAMA approved for the maintenance treatment of COPD in the US . Market research by Theravance Biopharma indicates approximately 9 % of the treated COPD patients in the US use nebulizers for ongoing maintenance therapy . 1 LAMAs are a cornerstone of maintenance therapy for COPD and YUPELRI ® is positioned as the first once - daily single - agent bronchodilator product for COPD patients who require, or prefer, nebulized therapy . YUPELRI ® ’s stability in both metered dose inhaler and dry powder device formulations suggest that this LAMA could also serve as a foundation for novel handheld combination products . 1. TBPH market research (N=160 physicians); refers to US COPD patients. 43

YUPELRI ® (revefenacin) inhalation solution YUPELRI ® inhalation solution is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD) . Important Safety Information (US) YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product . YUPELRI should not be initiated in patients during acutely deteriorating or potentially life - threatening episodes of COPD, or for the relief of acute symptoms, i . e . , as rescue therapy for the treatment of acute episodes of bronchospasm . Acute symptoms should be treated with an inhaled short - acting beta 2 - agonist . As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life - threatening . If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short - acting bronchodilator . YUPELRI should be discontinued immediately and alternative therapy should be instituted . YUPELRI should be used with caution in patients with narrow - angle glaucoma . Patients should be instructed to immediately consult their healthcare provider if they develop any signs and symptoms of acute narrow - angle glaucoma, including eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema . Worsening of urinary retention may occur . Use with caution in patients with prostatic hyperplasia or bladder - neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur . Immediate hypersensitivity reactions may occur after administration of YUPELRI . If a reaction occurs, YUPELRI should be stopped at once and alternative treatments considered . The most common adverse reactions occurring in clinical trials at an incidence greater than or equal to 2 % in the YUPELRI group, and higher than placebo, included cough, nasopharyngitis, upper respiratory infection, headache and back pain . Coadministration of anticholinergic medicines or OATP 1 B 1 and OATP 1 B 3 inhibitors with YUPELRI is not recommended . YUPELRI is not recommended in patients with any degree of hepatic impairment . OATP, organic anion transporting polypeptide. 44
