UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
Current Report Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event Reported): June 14, 2019
THERAVANCE BIOPHARMA, INC.
(Exact Name of Registrant as Specified in its Charter)
|
Cayman Islands |
|
001-36033 |
|
Not Applicable |
|
(State or Other Jurisdiction of |
|
(Commission File Number) |
|
(I.R.S. Employer Identification |
|
Incorporation) |
|
|
|
Number) |
PO Box 309
Ugland House, South Church Street
George Town, Grand Cayman, Cayman Islands KY1-1104
(650) 808-6000
(Addresses, including zip code, and telephone numbers, including area code, of principal executive offices)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading |
|
Name of each exchange |
|
Ordinary Share $0.00001 Par Value |
|
TBPH |
|
NASDAQ Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company o
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Item 7.01. Regulation FD Disclosure.
The information in this Current Report (including Exhibit 99.1) is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section. The information in this Current Report (including Exhibit 99.1) shall not be incorporated by reference into any registration statement or other document pursuant to the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
On June 14, 2019, members of the Theravance Biopharma, Inc. management team will be participating in a webinar hosted by Evercore ISI in New York, New York. A copy of the slide presentation is being furnished pursuant to Regulation FD as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
99.1 Slide deck entitled Investor Presentation - Evercore ISI Webinar- dated June 2019
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
THERAVANCE BIOPHARMA, INC. | |
|
|
|
|
|
Date: June 14, 2019 |
By: |
/s/ Bradford J. Shafer |
|
|
Bradford J. Shafer | |
|
|
Executive Vice President, General Counsel and Secretary | |
Theravance Biopharma, Inc. (NASDAQ: TBPH) Investor Presentation Evercore ISI Webinar June 14, 2019 © 2019 Theravance Biopharma. All rights reserved. THERAVANCE®, the Cross/Star logo, and MEDICINES THAT MAKE A DIFFERENCE® are registered trademarks of the Theravance Biopharma group of companies. YUPELRI® is a United States registered trademark of Mylan Specialty L.P. All third party trademarks used herein are the property of their respective owners.

Forward Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation may include the current dispute with Innoviva, Inc. and TRC LLC, statements relating to the company’s strategies, plans and objectives, the company’s regulatory strategies and timing of clinical studies (including the data therefrom), the potential characteristics, benefits and mechanisms of action of the company’s product and product candidates, the potential that the company’s research programs will progress product candidates into the clinic, the company’s expectations for product candidates through development, potential regulatory approval and commercialization (including their differentiation from other products or potential products), product sales or profit share revenue and the company’s expectations for its 2019 operating loss, excluding share-based compensation. The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to the nature of the current dispute with Innoviva and TRC LLC, the uncertainty of arbitration and litigation and the possibility that an arbitration award or litigation result involving the current dispute could be adverse to the company, delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 10, 2019, and other periodic reports filed with the SEC. 2

Transform the treatment of serious diseases with novel, locally acting organ-selective therapies 3 Theravance Biopharma Strategic Objective

4 Conventional Systemic Compound EFFECTIVE NON-EFFECTIVE DOSE-LIMITING SAFETY NON-EFFECTIVE DOSE-LIMITING SAFETY EFFECTIVE Theravance Biopharma Organ-selective Compound Illustrated example: lung selectivity Opportunity to increase dose for improved efficacy, without cost of systemic safety risk Expanded therapeutic index Often unable to achieve maximal efficacy due to dose-limiting safety Narrow therapeutic index Organ-selective Approach COMPOUNDS DESIGNED TO FULLY HARNESS INTENDED BIOLOGY

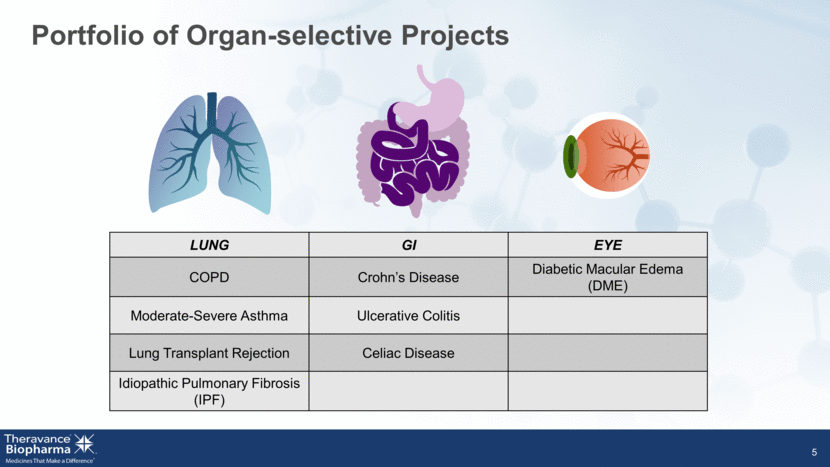
LUNG GI EYE COPD Crohn’s Disease Diabetic Macular Edema (DME) Moderate-Severe Asthma Ulcerative Colitis Lung Transplant Rejection Celiac Disease Idiopathic Pulmonary Fibrosis (IPF) 5 Portfolio of Organ-selective Projects
YUPELRI® (revefenacin) inhalation solution Nebulized long-acting muscarinic antagonist (LAMA)
YUPELRI®: Formal Commercial Launch Underway FDA-APPROVED FOR THE MAINTENANCE TREATMENT OF COPD 7 YUPELRI® (revefenacin) inhalation solution. Approved for the maintenance treatment of patients with COPD. COPD = Chronic Obstructive Pulmonary Disease. 1 Global Strategy for Diagnosis, Management, and Prevention of COPD. 2 Suboptimal Inspiratory Flow Rates Are Associated with COPD and All Cause Readmissions. Loh et al., Annals of ATS 2017. First and only once-daily bronchodilator delivered in a nebulizer Higher of two doses approved: 175 mcg once daily, for use with any standard jet nebulizer Unmet need for nebulized LAMA therapy Once-daily LAMAs are first-line therapy for moderate to severe COPD 1 No once-daily nebulized LAMAs available previously; only available in handheld devices Nebulized therapy associated with reduced hospital readmissions in low PIFR patients 2

Compelling Need for Once-Daily Nebulized LAMA ENDURING PATIENT NICHE AND SIGNIFICANT MARKET OPPORTUNITY 8 Enduring patient niche 9% of COPD patients currently use nebulizers for ongoing maintenance therapy 1 >100M patient treatment days in nebulized COPD segment 2 41% of COPD patients use nebulizers at least occasionally for bronchodilator therapy 1 Pricing in branded LA nebulized segment ~ 2x handheld Spiriva 2 Significant market opportunity YUPELRI® may be complementary to existing nebulized LABA treatments Mylan partnership brings commercial strength in nebulized segment 1 TBPH market research (N = 160 physicians); Refers to US COPD patients. 2 IMS Health information service: NSP for period MAT May, 2015. Excludes nebulized SABAs. IMS expressly reserves all rights, including rights of copying, distribution and republication.

Partnership with Mylan Brings Commercial Strength in Nebulized Opportunity 9 HD = hospital discharge HCPs = health care providers Targeting HCPs at key intersections in the patient’s disease management process Hospital is an important site of care for patients with worsening of COPD symptoms Theravance Biopharma’s established hospital-focused sales force is targeting the inpatient setting Theravance Biopharma partners with institutions to transition appropriate patients from hospital to home on YUPELRI® Mylan’s role is to ensure patients remain on YUPELRI® for maintenance therapy in the outpatient setting Combined sales infrastructures cover Hospital, Hospital Discharge and Home Health settings HD +

1 Global Strategy for Diagnosis, Management, and Prevention of COPD. 2 GOLD 2019. 3 Tashkin 2017. Use of YUPELRI® Consistent with GOLD Guidelines LAMAs RECOMMENDED AS FIRST-LINE TREATMENT 1 Use of a nebulized treatment is optimal for many COPD patients At least 2/3 of patients make at least one error using an inhaler device, many critical to delivery of medication 2 Main errors in delivery device use relate to problems with inspiratory flow, inhalation duration, coordination, etc. Choice of inhaler depends on access, cost, prescriber and most importantly, patient ability and preference COPD is associated with many risk factors for difficulty using an inhaler device 3 YUPELRI®: First and only once-daily nebulized LAMA Older age and arthritic hands Dyspnea which makes breath holding challenging Muscle weakness and impaired diaphragm function which can limit inspiratory force Cognitive dysfunction and dementia which increases the risk of incorrect MDI/DPI use Once-daily dosing (via any standard jet nebulizer) Proven 24-hour control Demonstrated safety profile Up to 100% of Medicare Part B patients covered 10

PIFR is the measure of patient inspiratory effort during a maximal effort Effective DPI use requires patients generate sufficient inspiratory flow rate to overcome internal resistance of a particular devise in order to deaggregate powdered drug into fine particles for optimal peripheral airway and lung deposition Suboptimal PIFR has been observed in significant proportion of patients with COPD Patients with suboptimal PIFR also associated with: 1Mahler ATS 2017; 2Loh et al 2018; 3 Mahler ATS 2019 Insufficient Peak Inspiratory Flow Rate (PIFR) Associated with Increased Burden More respiratory infections in previous 12 months Smaller lung size (female, shorter height) Lower lung function (% Predicted FVC) Increased risk of re-hospitalization 2 More severe symptoms 3 19%–25% of stable outpatients 1 32%–52% of inpatients pre-discharge for COPD exacerbation 2 Optimal PIFR Sub-optimal PIFR Total (N = 252) GOLD 2 (n = 159) GOLD 3 (n = 78) GOLD 4 (n = 15) Total (N = 273) GOLD 2 (n = 78) GOLD 3 (n = 82) GOLD 4 (n = 113) mMRC 2, n (%) 123 (49) 71 (45) 40 (51) 12 (80) 191 (70) 40 (51) 59 (72) 92 (81) mMRC, mean (SD) 1.6 (1.0) 1.4 (1.0) 1.7 (0.9) 2.1 (0.8) 2.1 (1.0) 1.6 (0.9) 2.1 (1.0) 2.4 (0.9) BDI, mean (SD) 6.1 (2.0) 6.3 (2.0) 5.6 (1.8) 5.4 (1.8) 5.1 (2.0) 5.9 (2.1) 5.0 (1.9) 4.7 (1.7) Mahler et al ATS 2019 11

YUPELRI® Launch Update ENCOURAGING INITIAL MARKET RESPONSE 12 32 Wins (equates to 104 accounts) ~80 Reviews Scheduled (~320 potential accounts) 100% medical support requests fulfilled <30 days FORMULARY Field force productivity goals exceeded ~4,500 patients prescribed (thru 1Q19) PATIENT 100% Medicare Part B 1 ~50% Commercial Permanent J-CODE issued (effective July 1, 2019) ACCESS 12 Majority of YUPELRI® volume flows through durable medical equipment (DME) channel 2; remaining volume flows through hospitals, retail and long-term care pharmacies WAC: $1,030 per month (or ~$34 per day) 1 For patients with supplemental insurance 2 Approximately 3 month lag in data capture

Opportunity for YUPELRI® (revefenacin) in China POTENTIAL TO ADDRESS LARGE AND UNDERSERVED COPD PATIENT POPULATION 13 18 Expansion of development and commercialization agreement Mylan granted exclusive development and commercialization rights to revefenacin in China and adjacent territories Theravance Biopharma eligible to receive: $18.5 million upfront payment Up to $54 million in additional potential development and sales milestones Tiered royalties on net sales, if approved Mylan responsible for all aspects of development and commercialization in partnered regions Significant market opportunity COPD affects ~100 million individuals in China1 ~43% of COPD patients suffer from moderate to very severe forms of disease2 COPD is one of the top three causes of death in China3 and presents significant financial burden to healthcare system2 Theravance Biopharma and Mylan strategic collaboration In 2015, the companies established a strategic collaboration to develop and commercialize nebulized revefenacin products for COPD and other respiratory diseases Theravance Biopharma eligible to receive up to $259 million in potential development and sales milestone payments, as well as profit-sharing arrangement with Mylan on US sales and tiered royalties on ex-US sales Theravance Biopharma retains worldwide rights delivered through other dosage forms, including metered dose inhaler and dry powder inhaler (MDI/PDI) 1C. Wang, J. Xu, L. Yang et al., “Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study,” The Lancet, vol. 391, no. 10131, pp. 1706–1717, 2018. 2 Fang L, Gao P, Bao H, et al., “Chronic obstructive pulmonary disease in China: a nationwide prevalence study,” Lancet Respir Med 2018; 6: 421–430. 3 Yin P, Wang H, Vos T, et al., “A subnational analysis of mortality and prevalence of COPD in China From 1990 to 2013: Findings from the global burden of disease study 2013,” Chest. 2016;150:1269–1280.

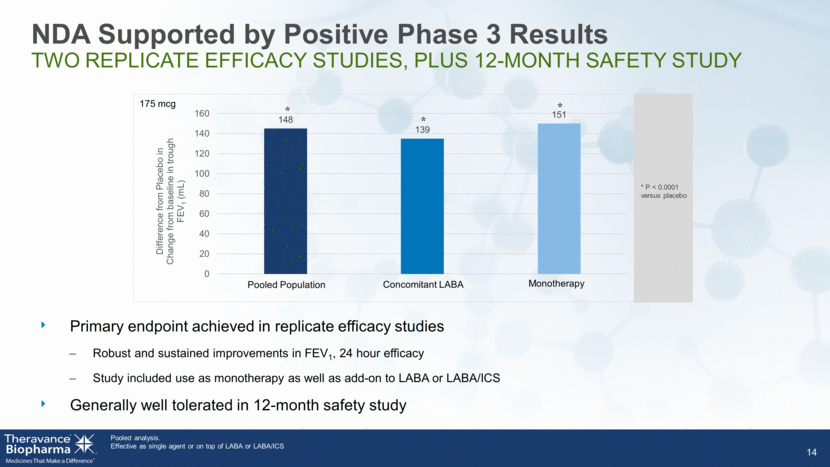
NDA Supported by Positive Phase 3 Results TWO REPLICATE EFFICACY STUDIES, PLUS 12-MONTH SAFETY STUDY 14 Pooled analysis. Effective as single agent or on top of LABA or LABA/ICS Primary endpoint achieved in replicate efficacy studies Robust and sustained improvements in FEV1, 24 hour efficacy Study included use as monotherapy as well as add-on to LABA or LABA/ICS Generally well tolerated in 12-month safety study * * * Monotherapy Concomitant LABA Pooled Population * P < 0.0001 versus placebo 175 mcg
Consistent Treatment Effect Maintained for 24 Hours with QD Dosing in Phase 3 Efficacy 15 Source: Phase 3 efficacy studies of revefenacin (TD-4208). PD: pre-dose. Note: 24-hour spirometry was assessed at baseline and after 12 weeks’ treatment in a subset of subjects in both studies. Dose dependent effect on FEV1 with 175 consistently better than 88 mcg (N=92) (N=89)

Low Incidence of Adverse Events and Comparable to Placebo in Phase 3 Efficacy 16 Source: Phase 3 efficacy studies of revefenacin (TD-4208). 1 Sudden death in placebo group attributed as cardiovascular by independent adjudication. n=1229 unique subjects; 1 subject was randomized to 175 mcg but also received placebo; this subject is counted in both groups in the safety population Description Placebo (N=418) 88mcg (N=417) 175mcg (N=395) Serious Adverse Events 21 (5%) 21 (5%) 15 (4%) Deaths: - Homicide 0 (0%) 0 (0%) 1 (0.2%) - Sudden death1 1 (0.2%) 0 (0%) 0 (0%) Adverse Events (AEs) 206 (49%) 226 (54%) 203 (51%) Possibly/probably Related AEs 39 (9%) 33 (8%) 41 (10%) AEs Leading to Study Drug Discontinuation 59 (14%) 50 (12%) 43 (11%)

Most Frequently Reported Adverse Events (AEs) in Phase 3 Efficacy Studies 17 Source: Phase 3 efficacy studies of revefenacin (TD-4208). Description Placebo (N=418) 88 mcg (N=417) 175 mcg (N=395) Exacerbation of COPD 48 (11.5%) 42 (10.1%) 42 (10.6%) Cough 17 (4.1%) 17 (4.1%) 17 (4.3%) Dyspnea 23 (5.4%) 13 (3.1%) 12 (3.0%) Headache 11 (2.6%) 21 (5.0%) 16 (4.1%) n=1229 unique subjects; 1 subject was randomized to 175 mcg but also received placebo; this subject is counted in both groups in the safety population No reports of worsening of urinary retention, blurred vision or narrow-angle glaucoma Dry mouth reported in <0.5% of patients on revefenacin

TD-1473 JAK Inhibitor Program Investigational oral gut-selective pan-Janus kinase (JAK) inhibitor for ulcerative colitis and other inflammatory intestinal diseases
Gut-selective Design INFLAMMATION TREATED AT THE TISSUE OF INTEREST 19 Metabolism Gut-selective JAK inhibitor Absorption Metabolism Absorption Systemic JAK inhibitor To feces Gut wall & mucosa Liver Portal vein Intestine To feces Gut wall & mucosa Liver Portal vein Intestine Systemic Circulation Systemic Circulation Systemically available drug eliminated by the liver via first pass metabolism

TD-1473: Innovative Approach in Treating IBD compelling Preclinical PACKAGE AND ENCOURAGING Phase 1b DATA 20 Gut selectivity Potent inhibition of Tyk2 Anti-inflammatory activity in disease model Lumen Vehicle treated control TD-1473 treatment Inhibitory potency (pKi) Tyk2 7.5 8 8.5 9 9.5 10 Tofacitinib TD-1473

Differentiated and Potential Breakthrough Approach ADVANCING IN COLLABORATION WITH JANSSEN IN UC AND CROHN’S 21 TD-1473 program objectives: Oral pan-JAK inhibitor that distributes selectively throughout the intestines to treat inflammatory intestinal disease locally, with minimal systemic exposure or corresponding immunosuppressive effects, to enhance safety and efficacy Encouraging Phase 1b study in UC patients Data demonstrated localized biological target engagement with minimal systemic exposure Clinical responses after only 4 weeks of therapy Preclinical models of UC confirmed Improvements in diseases scores, local absorption and penetration of TD-1473 throughout intestinal tract Phase 2 in Crohn’s progressing and Phase 2b/3 study in UC underway FDA and EMA concur on Phase 2b/3 study design in ulcerative colitis UC: ulcerative colitis.

Encouraging Findings in Phase 1b Study 4-WEEK TREATMENT IN 40 PATIENTS WITH ULCERATIVE COLITIS 22 Detailed results presented in oral late-breaker at UEGW 2018 1 Clinical response as measured by both partial and full Mayo. 2 Surrogate biomarkers include C-reactive protein (CRP) and fecal calprotectin. Key Findings Favorable overall safety and tolerability No systemic or opportunistic infections (including herpes zoster) No evidence of reduce white cell counts Minimal systemic exposure Plasma levels of TD-1473 very low Consistent in all cohorts to levels observed in healthy volunteers Biologic activity in GI tract Endoscopic improvements and mucosal healing reported in all active arms; none reported in placebo arm Rectal bleeding scores improved above placebo at highest two doses Rates of clinical response higher for all active doses compared to placebo1 Clinical responses matched by dose-dependent reductions in surrogate biomarkers2 Dose-related increases in local GI tissue drug concentrations; higher two doses produced mean concentrations above JAK IC50

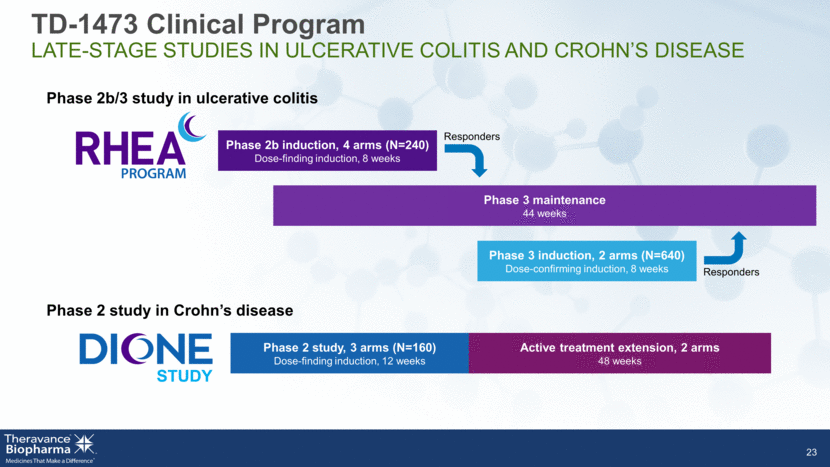
Phase 3 induction, 2 arms (N=640) Dose-confirming induction, 8 weeks Phase 2b induction, 4 arms (N=240) Dose-finding induction, 8 weeks 23 Phase 3 maintenance 44 weeks Phase 2b/3 study in ulcerative colitis Phase 2 study in Crohn’s disease Phase 2 study, 3 arms (N=160) Dose-finding induction, 12 weeks Active treatment extension, 2 arms 48 weeks Responders Responders TD-1473 Clinical Program LATE-STAGE STUDIES IN ULCERATIVE COLITIS AND CROHN’S DISEASE
Global Collaboration Agreement for TD-1473 PURPOSED TO MAXIMIZE VALUE OF PROGRAM 24 Shared belief in TD-1473 as gut-selective with potential to transform the treatment landscape in inflammatory intestinal disease Meaningful program enhancements Accelerate clinical development and advance UC and Crohn’s in parallel Apply Janssen expertise in IBD to optimize clinical strategy and execution Maximize worldwide commercial opportunity Attractive deal economics reducing overall financial risk Deal value up to $1B milestones, including $100M upfront; additional profit-share in US Collaboration with global leader in immunology represents milestone for TD-1473, our internally discovered pipeline and strategy to design organ-selective medicines

Ampreloxetine (TD-9855) Investigational once-daily norepinephrine reuptake inhibitor (NRI) for neurogenic orthostatic hypotension (nOH)

26 1 Existing options associated with one or both therapies noted above; Northera prescribing information. MSA: multiple system atrophy. PD: Parkinson’s disease. PAF: pure autonomic failure. TID: three times per day. nOH characterized by a sustained drop in blood pressure upon standing, due to body producing insufficient levels of norepinephrine (NE) Associated with several autonomic disorders: MSA, PD, PAF Symptoms include dizziness, fainting, blurred vision and weakness Orphan indication with < 200k patients in US Opportunity exists for safe and effective treatment Only droxidopa (Northera) and midodrine FDA-approved for nOH Synthetic exogenous NE analogues impact disease by increasing vascular tone Limitations of current therapy: Supine hypertension, TID dosing, patients refractory or discontinue, lack of durability1 Ideal therapy would target durable improvement in symptoms and daily function NE NE NE NE NET NET NE Vasodilation lowers blood pressure Blood pressure key biological driver to nOH symptoms Symptomatic nOH Represents a Significant Unmet Need

NET Inhibition with Ampreloxetine Offers Potential to Restore Vascular Sympathetic Tone 27 1 Includes Phase 1 SAD/MAD, elderly, and PET studies in healthy subjects and Phase 2a studies in fibromyalgia and ADHD patients. NET: norepinephrine transporter. QD: once-daily. A path to treating symptomatic nOH without introducing exogenous NE Blockade of NET in nOH patients inhibits endogenous neuronal NE uptake Increased levels of NE in the synapse cause vasoconstriction and a corresponding increase in blood pressure Increase in blood pressure improves symptoms Rationale for ampreloxetine in nOH NRI with NE dominance confirmed in humans QD dosing, long half-life, and metabolic profile for potential improved patient outcomes Favorable safety and tolerability profile established in > 500 subjects1 Vasoconstriction to increase blood pressure + Ampreloxetine NE NE NE NE NET NET NE NE NE

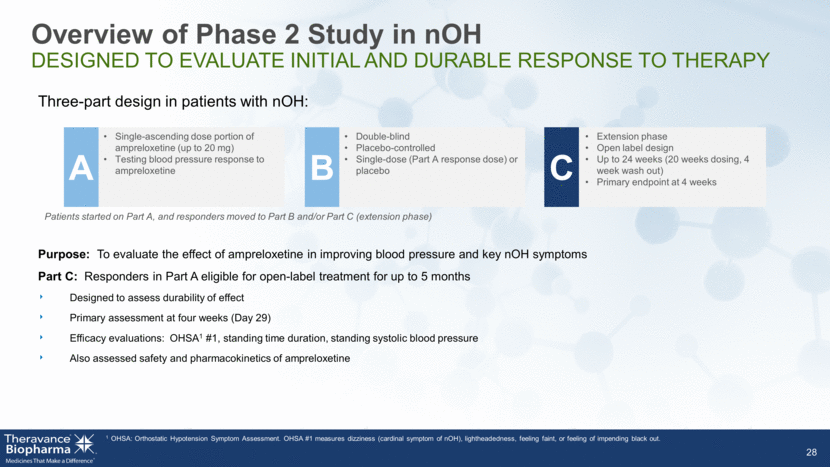
Overview of Phase 2 Study in nOH DESIGNED TO EVALUATE INITIAL AND DURABLE RESPONSE TO THERAPY 28 Purpose: To evaluate the effect of ampreloxetine in improving blood pressure and key nOH symptoms Part C: Responders in Part A eligible for open-label treatment for up to 5 months Designed to assess durability of effect Primary assessment at four weeks (Day 29) Efficacy evaluations: OHSA1 #1, standing time duration, standing systolic blood pressure Also assessed safety and pharmacokinetics of ampreloxetine 1 OHSA: Orthostatic Hypotension Symptom Assessment. OHSA #1 measures dizziness (cardinal symptom of nOH), lightheadedness, feeling faint, or feeling of impending black out. Three-part design in patients with nOH: Single-ascending dose portion of ampreloxetine (up to 20 mg) Testing blood pressure response to ampreloxetine A Patients started on Part A, and responders moved to Part B and/or Part C (extension phase) Double-blind Placebo-controlled Single-dose (Part A response dose) or placebo B Extension phase Open label design Up to 24 weeks (20 weeks dosing, 4 week wash out) Primary endpoint at 4 weeks C
29 Part B change from baseline systolic blood pressure (n=10) LS Mean+ / - SE Change from Baseline (mmHg) Top-line Phase 2 Results in nOH PARTS A and B: SINGLE-ASCENDING DOSE, AMPRELOXETINE OR PLACEBO Initial responses observed A Responses reported in majority of patients treated 27 of 34 patients enrolled in Part A showed improvements in SBP and/or standing time Responses observed above 5 mg Confirmation vs. placebo B Statistically significant difference of 30 mmHg at 4 hours post-dose (p = 0.011) Ampreloxetine increased SBP from a low baseline SBP dropped on placebo during day as expected, due to postural changes and eating No evidence of supine hypertension with ampreloxetine overnight SBP: systolic blood pressure.

30 Top-line Phase 2 Results in nOH PART C: REPEAT DOSE EXTENSION PHASE Durability of effect observed out to 4 weeks C 16 of 21 patients (76%) completed four weeks of treatment Reductions in symptom severity, with most pronounced benefit in patients with symptomatic nOH1 Mean reduction in OHSA #1 = 2.4 points at four weeks (n=16) 13 completers had OHSA #1 > 4 points at baseline; mean reduction in group = 3.8 points at four weeks Consistent increases in SBP through four weeks Clinically meaningful increases in standing SBP (7 mmHg or greater) after standing for three minutes at all time points on all weekly clinic visits Generally well tolerated; no serious adverse events assessed as drug-related Registrational Phase 3 program in symptomatic nOH progressing 1 Symptomatic defined as OSHA #1 > 4.

Ampreloxetine Clinical Program PHASE 3 REGISTRATIONAL PROGRAM IN SYMPTOMATIC NOH 31 De Novo Study 0169 4 weeks (N=188) Randomized, double-blind, placebo-controlled, parallel group study Study 0170 22 weeks (N=258) Randomized 6-week withdrawal phase (preceded by 4-month open label) Supportive 5-month treatment data from Phase 2 study to be presented at IAPRD and ENC

TD-8236 Investigational, lung-selective inhaled JAK inhibitor for moderate-to-severe asthma regardless of Th2 phenotype

33 Small portion of US patients cause high proportion of cost Severe 14% Moderate 16% Severe 61% Moderate 25% 16M diagnosed asthma cases1 Healthcare utilization2 Patient population 4.9M moderate-to-severe diagnosed patients in US1 Current treatments Inhaled steroids, which often fail to control disease Approved biologics affect subsets of patients Burden of disease Acute exacerbations lead to ER visits Uncontrolled symptoms interfere with ability to sleep, work and QOL US medical costs estimated to be $58B3 Disproportionate healthcare utilization by severe and uncontrolled asthmatics High frequency of hospitalizations and increased use of systemic medications 1 © 2018 DR/Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. 2 Sadatsafavi, M., et al. Can Respir J, 2010 17(2): 74-80. 3 Nurmagambetov, T., et al., Ann Am Thorac Soc. 2018. High Medical and Economic Burden in Uncontrolled Asthma

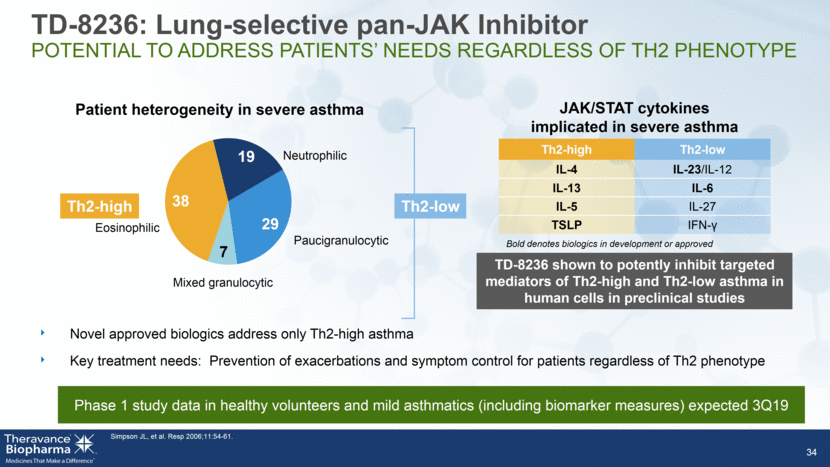
JAK/STAT cytokines implicated in severe asthma Th2-high Th2-low IL-4 IL-23/IL-12 IL-13 IL-6 IL-5 IL-27 TSLP IFN- 34 Mixed granulocytic Eosinophilic Neutrophilic Paucigranulocytic Patient heterogeneity in severe asthma Th2-low Th2-high Bold denotes biologics in development or approved Novel approved biologics address only Th2-high asthma Key treatment needs: Prevention of exacerbations and symptom control for patients regardless of Th2 phenotype Simpson JL, et al. Resp 2006;11:54-61. TD-8236 shown to potently inhibit targeted mediators of Th2-high and Th2-low asthma in human cells in preclinical studies TD-8236: Lung-selective pan-JAK Inhibitor POTENTIAL TO ADDRESS PATIENTS’ NEEDS REGARDLESS OF TH2 PHENOTYPE Phase 1 study data in healthy volunteers and mild asthmatics (including biomarker measures) expected 3Q19 38 19 29 7
Economic Interest GSK’s FDA-approved TRELEGY ELLIPTA (FF/UMEC/VI): First and only once-daily single inhaler triple therapy

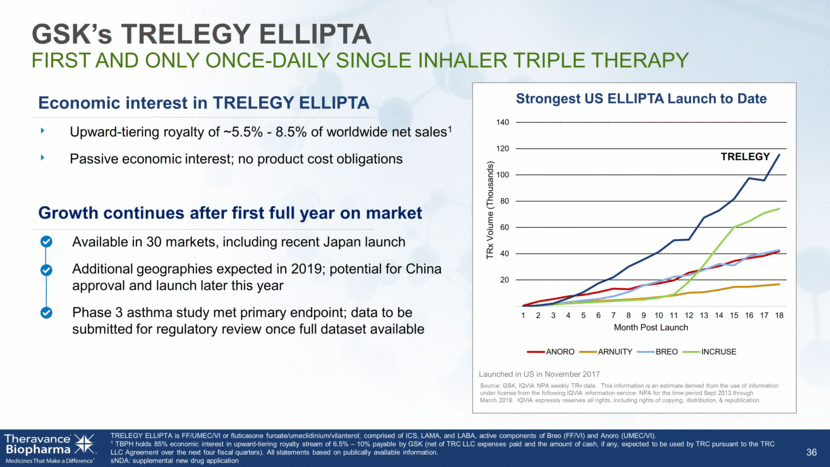
GSK’s TRELEGY ELLIPTA FIRST AND ONLY ONCE-DAILY SINGLE INHALER TRIPLE THERAPY 36 Economic interest in TRELEGY ELLIPTA Upward-tiering royalty of ~5.5% - 8.5% of worldwide net sales1 Passive economic interest; no product cost obligations Growth continues after first full year on market Available in 30 markets, including recent Japan launch Additional geographies expected in 2019; potential for China approval and launch later this year Phase 3 asthma study met primary endpoint; data to be submitted for regulatory review once full dataset available TRELEGY ELLIPTA is FF/UMEC/VI or fluticasone furoate/umeclidinium/vilanterol; comprised of ICS, LAMA, and LABA, active components of Breo (FF/VI) and Anoro (UMEC/VI). 1 TBPH holds 85% economic interest in upward-tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC LLC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC LLC Agreement over the next four fiscal quarters). All statements based on publically available information. sNDA: supplemental new drug application 20 40 60 80 100 120 140 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 TRx Volume (Thousands) Month Post Launch Strongest US ELLIPTA Launch to Date ANORO ARNUITY BREO INCRUSE TRELEGY Launched in US in November 2017 Source: GSK, IQVIA NPA weekly TRx data. This information is an estimate derived from the use of information under license from the following IQVIA information service: NPA for the time period Sept 2013 through March 2019. IQVIA expressly reserves all rights, including rights of copying, distribution, & republication.
Non-recourse PhaRMASM 9% Fixed Rate Term Notes SECURITIZED BY ROYALTIES DUE ON NET SALES OF TRELEGY ELLIPTA 37 TRELEGY ELLIPTA is FF/UMEC/VI or fluticasone furoate/umeclidinium/vilanterol; comprised of ICS, LAMA, and LABA, active components of Breo (FF/VI) and Anoro (UMEC/VI). 1Net proceeds (excluding $12.5 million of retained notes and other fees related to the transaction) were approximately $230 million 2TBPH holds 85% economic interest in upward-tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC LLC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC LLC Agreement over the next four fiscal quarters). Transaction generated cash with retained economics December 2018 TBPH executed a non-dilutive private placement of $250M1 PhaRMASM 9% fixed rate term notes Debt payable by economic interest in TRELEGY ELLIPTA2 75% of royalties pledged to repay debt 25% of royalties retained by TBPH Proceeds support key strategic priorities Following repayment of notes, all TRELEGY ELLIPTA related cash flows revert to TBPH Non-recourse notes with flexible repayment terms Notes fully securitized by royalties due on net sales of TRELEGY ELLIPTA; no debt obligation to TBPH Quarterly interest obligations: Through October 15, 2020, to the extent there are insufficient funds to satisfy quarterly interest payments, interest may be paid in-kind without a default or event of default occurring; or At TBPH’s option, quarterly interest payments may be satisfied by making a capital contribution for no more than 4 consecutive quarterly interest payment dates or for no more than 6 quarterly interest payment dates during the term of the notes

Arbitration Against Innoviva, Inc. (INVA) TBPH INTENDS TO ENFORCE ALL RIGHTS TO TRELEGY ELLIPTA ROYALTIES1 38 TRELEGY ELLIPTA is FF/UMEC/VI or fluticasone furoate/umeclidinium/vilanterol; comprised of ICS, LAMA, and LABA, active components of Breo (FF/VI) and Anoro (UMEC/VI). 1TBPH holds 85% economic interest in upward-tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC LLC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC LLC Agreement over the next four fiscal quarters). The Respiratory Company, LLC (TRC LLC) Upon our spin-off from INVA in 2014, TBPH and INVA entered into a binding limited liability company agreement TRC LLC is jointly owned by TBPH and INVA but managed by INVA TBPH entitled to 85% and INVA entitled to 15% of royalties paid to TRC LLC by GSK resulting from GSK’s net sales of TRELEGY ELLIPTA Agreement imposes express fiduciary duties on INVA and significant limitations on INVA’s authority as manager Arbitration underway In May 2019, we initiated arbitration against INVA and TRC LLC due to INVA’s failure to disburse stipulated royalties to TBPH INVA has caused TRC LLC to not remit royalty payments for 4Q18; and INVA has stated it intends to cause TRC LLC to withhold cash distributions for the remainder of 2019 TBPH intends to enforce all aspects of the agreement to ensure we continue receiving our 85% share of TRELEGY-related royalties Agreement stipulates parties shall use commercially reasonable best efforts to complete arbitration within 90 days after the arbitrator(s) is/are appointed We are confident that the arbitration process provides an expedient forum to resolving the dispute in our favor Confidentiality provisions limit what we can communicate publicly about this matter. We have and will continue to abide by those confidentiality provisions, which require all parties to keep this matter confidential, subject to any non-waivable disclosure obligations under applicable law

Opportunities for Value Creation Upcoming milestones

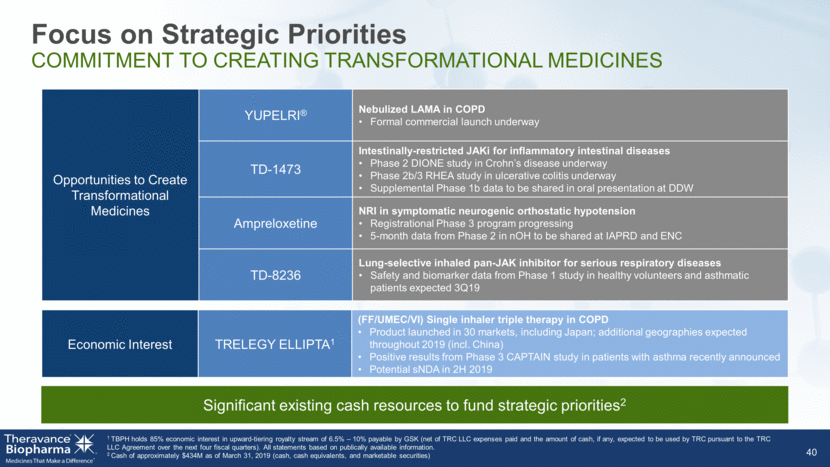
Significant existing cash resources to fund strategic priorities2 Focus on Strategic Priorities COMMITMENT TO CREATING TRANSFORMATIONAL MEDICINES 40 1 TBPH holds 85% economic interest in upward-tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC LLC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC LLC Agreement over the next four fiscal quarters). All statements based on publically available information. 2 Cash of approximately $434M as of March 31, 2019 (cash, cash equivalents, and marketable securities) Opportunities to Create Transformational Medicines YUPELRI® Nebulized LAMA in COPD Formal commercial launch underway TD-1473 Intestinally-restricted JAKi for inflammatory intestinal diseases Phase 2 DIONE study in Crohn’s disease underway Phase 2b/3 RHEA study in ulcerative colitis underway Supplemental Phase 1b data to be shared in oral presentation at DDW Ampreloxetine NRI in symptomatic neurogenic orthostatic hypotension Registrational Phase 3 program progressing 5-month data from Phase 2 in nOH to be shared at IAPRD and ENC TD-8236 Lung-selective inhaled pan-JAK inhibitor for serious respiratory diseases Safety and biomarker data from Phase 1 study in healthy volunteers and asthmatic patients expected 3Q19 Economic Interest TRELEGY ELLIPTA1 (FF/UMEC/VI) Single inhaler triple therapy in COPD Product launched in 30 markets, including Japan; additional geographies expected throughout 2019 (incl. China) Positive results from Phase 3 CAPTAIN study in patients with asthma recently announced Potential sNDA in 2H 2019
About YUPELRI® (revefenacin) inhalation solution YUPELRI® (revefenacin) inhalation solution is a novel once-daily nebulized LAMA approved for the maintenance treatment of COPD in the US. Market research by Theravance Biopharma indicates approximately 9% of the treated COPD patients in the US use nebulizers for ongoing maintenance therapy.1 LAMAs are a cornerstone of maintenance therapy for COPD and YUPELRI is positioned as the first once-daily single-agent bronchodilator product for COPD patients who require, or prefer, nebulized therapy. YUPELRI’s stability in both metered dose inhaler and dry powder device formulations suggest that this LAMA could also serve as a foundation for novel handheld combination products. 1 TBPH market research (N = 160 physicians); refers to US COPD patients 41

YUPELRI® (revefenacin) inhalation solution Important Safety Information (US) YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product. YUPELRI should not be initiated in patients during acutely deteriorating or potentially life-threatening episodes of COPD, or for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Acute symptoms should be treated with an inhaled short-acting beta2-agonist. As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short-acting bronchodilator. YUPELRI should be discontinued immediately and alternative therapy should be instituted. YUPELRI should be used with caution in patients with narrow-angle glaucoma. Patients should be instructed to immediately consult their healthcare provider if they develop any signs and symptoms of acute narrow-angle glaucoma, including eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema. Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur. Immediate hypersensitivity reactions may occur after administration of YUPELRI. If a reaction occurs, YUPELRI should be stopped at once and alternative treatments considered. The most common adverse reactions occurring in clinical trials at an incidence greater than or equal to 2% in the YUPELRI group, and higher than placebo, included cough, nasopharyngitis, upper respiratory infection, headache and back pain. Coadministration of anticholinergic medicines or OATP1B1 and OATP1B3 inhibitors with YUPELRI is not recommended. YUPELRI is not recommended in patients with any degree of hepatic impairment. 42

