Theravance Biopharma, Inc. Announces Results from Study 0170, a Second Phase 3 Study of Ampreloxetine, in Patients with Symptomatic Neurogenic Orthostatic Hypotension (nOH)
- Results from Study 0170 show a benefit in study patients with multiple system atrophy (MSA)
- Company beginning discussions with potential strategic partners and planning health authority interactions to expedite ampreloxetine as a possible treatment option for patients with symptomatic nOH
- Company remains focused on respiratory therapeutics, value creation for shareholders and reiterates plan to be sustainably cash-flow positive by the second half of the year
The primary endpoint was not statistically significant for the overall population of patients which included patients with Parkinson's disease (PD), pure autonomic failure (PAF) and MSA (odds ratio=0.6; p-value=0.196). The odds ratio suggests that patients receiving ampreloxetine had a 40% reduction in the odds of treatment failure compared to placebo.
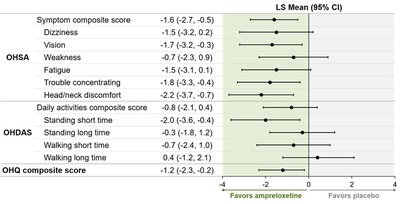
The pre-specified subgroup analysis by disease type suggests the benefit seen in patients receiving ampreloxetine was largely driven by MSA patients (n=40). An odds ratio of 0.28 (95% CI: 0.05, 1.22) was observed in MSA patients indicating a 72% reduction in the odds of treatment failure with ampreloxetine compared to placebo. The benefit to MSA patients was observed in multiple endpoints including OHSA composite, Orthostatic Hypotension Daily Activities Scale (OHDAS) composite, Orthostatic Hypotension Questionnaire (OHQ) composite and OHSA #1 (see figure below). Notably, patients withdrawn to placebo had a clinically relevant decrease in standing blood pressure; there was no decrease for patients remaining on ampreloxetine. While the same benefit was not apparent in patients with PD or PAF, the Company continues to analyze the data to better understand this observation. Throughout the study, there was no indication of worsening of supine hypertension based on 24-hour monitoring. Data suggest that ampreloxetine was well-tolerated and no new safety signals were identified.
"MSA is a devastating and debilitating disease with no effective disease modifying treatment. There is an urgency to treat MSA patients suffering with nOH due to the impact on quality of life and the extreme caregiver burden. Ampreloxetine appears to improve a narrow, but critically important group of symptoms related to blood pressure control, and along with the safety profile, may represent a potential therapy for MSA patients," said
"At Theravance Biopharma, we are guided by patient outcomes. Given the clear unmet need for MSA patients suffering from symptomatic nOH, we are engaging potential partners and planning health authority interactions to determine a path forward in hopes of expediting ampreloxetine as a possible treatment option for people with MSA," said Rick E Winningham, Chief Executive Officer, Theravance Biopharma. "Concurrently, we are executing against our business plan and remain disciplined in capital allocation, maximizing shareholder value and re-iterate our goal to be sustainably cash-flow positive by the second half of this year."
Disclosure:
Study 0170 (REDWOOD) Ampreloxetine Phase 3 Results
About the Phase 3 Study
Study 0170 (NCT03829657) was a 22-week Phase 3 study comprised of a 16-week open-label period and a 6-week double-blind, placebo-controlled, randomized withdrawal period. The primary endpoint of treatment failure at week 6 was defined as a worsening of both Orthostatic Hypotension Symptom Assessment Scale (OHSA) question #1 and Patient Global Impression of Severity (PGI-S) scores by 1.0 point. After Study 0169 did not meet its primary endpoint, the Company took actions to close out the ongoing clinical program including Study 0170. The study was more than 80% enrolled (n=128/154 planned) despite stopping early.
About Symptomatic nOH
Neurogenic orthostatic hypotension (nOH) is a rare disorder defined as a sustained orthostatic fall in systolic blood pressure (SBP) of ≥ 20 mm Hg or diastolic blood pressure (DBP) of ≥ 10 mm Hg within three minutes of standing. Severely affected patients are unable to stand for more than a few seconds because of their decrease in blood pressure, leading to cerebral hypoperfusion and syncope. A debilitating condition, nOH results in a range of symptoms including dizziness, lightheadedness, fainting, fatigue, blurry vision, weakness, trouble concentrating, and head and neck pain. nOH is caused by autonomic nervous system malfunction and is associated with several underlying medical conditions including multiple system atrophy (MSA), pure autonomic failure (PAF), and Parkinson's disease (PD).
About Multiple System Atrophy (MSA)
MSA (formerly Shy-Drager syndrome) is a rare, rapidly progressive, severely debilitating, and fatal neurodegenerative disease that leads to death within a median of seven to 10 years after the onset of symptoms. MSA shares many Parkinson's disease-like symptoms, such as slow movement, rigid muscles and poor balance. The primary sign of multiple system atrophy is postural (orthostatic) hypotension. A person with MSA can also develop dangerously high blood pressure levels while lying down (supine hypertension). Other manifestations include: urinary and bowel dysfunction, constipation, incontinence, sweating abnormalities, sleep disorders, sexual dysfunction, cardiovascular problems and psychiatric problems. The most common causes of death in MSA are infection and cardiopulmonary complications. Currently, MSA patients receive only symptomatic and palliative therapies as there are no disease-modifying treatments and no cure.
About Ampreloxetine
Ampreloxetine (TD-9855) is an investigational, Theravance Biopharma-discovered, potent, long-acting, oral, once-daily norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) with patent protection until 2037 in the US. In addition to the Phase 3 clinical program,
About
In pursuit of its purpose, Theravance Biopharma leverages decades of respiratory expertise to discover and develop transformational medicines that make a difference. These efforts have led to the development of FDA-approved YUPELRI® (revefenacin) inhalation solution indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Its respiratory pipeline of internally discovered programs is targeted to address significant patient respiratory needs.
Theravance Biopharma has an economic interest in potential future payments from Glaxo Group Limited or one of its affiliates (GSK) pursuant to its agreements with Innoviva, Inc. relating to certain programs, including TRELEGY.
For more information, please visit www.theravance.com.
YUPELRI® is a registered trademark of Mylan Specialty L.P., a Viatris Company. Trademarks, trade names or service marks of other companies appearing on this press release are the property of their respective owners.
Forward-Looking Statements
This press release contains certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives, expectations and future events. Theravance Biopharma intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements relating to: the Company's goals, designs, strategies, plans and objectives, ability to provide value to shareholders, the Company's regulatory strategies, the potential characteristics, benefits and mechanisms of action of the Company's product and product candidates, the Company's expectations for product candidates through development and the market for products being commercialized, the Company's expectations regarding its allocation of resources, potential regulatory actions and commercialization, product sales or profit share revenue and the Company's expectations for its expenses, excluding share-based compensation and other financial results. These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of the press release and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopharma to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: additional future analysis of the data resulting from our clinical trial(s), the potential that results from clinical or non-clinical studies indicate the Company's compounds, products or product candidates are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable to the Company, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and ability to retain key personnel, the impact of the Company's restructuring actions on its employees, partners and others. In addition, while we expect the effects of COVID-19 to continue to adversely impact our business operations and financial results, the extent of the impact on our ability to generate revenue from YUPELRI® (revefenacin), our clinical development programs, and the value of and market for our ordinary shares, will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time. Other risks affecting Theravance Biopharma are in the Company's Form 10-K filed with the SEC on February 28, 2022, and other periodic reports filed with the SEC. In addition to the risks described above and in Theravance Biopharma's filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma's results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance Biopharma assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law.
Contact: Gail B. Cohen
Corporate Communications / 917-214-6603
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/theravance-biopharma-inc-announces-results-from-study-0170-a-second-phase-3-study-of-ampreloxetine-in-patients-with-symptomatic-neurogenic-orthostatic-hypotension-noh-301517024.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/theravance-biopharma-inc-announces-results-from-study-0170-a-second-phase-3-study-of-ampreloxetine-in-patients-with-symptomatic-neurogenic-orthostatic-hypotension-noh-301517024.html
SOURCE